Safe PBM study
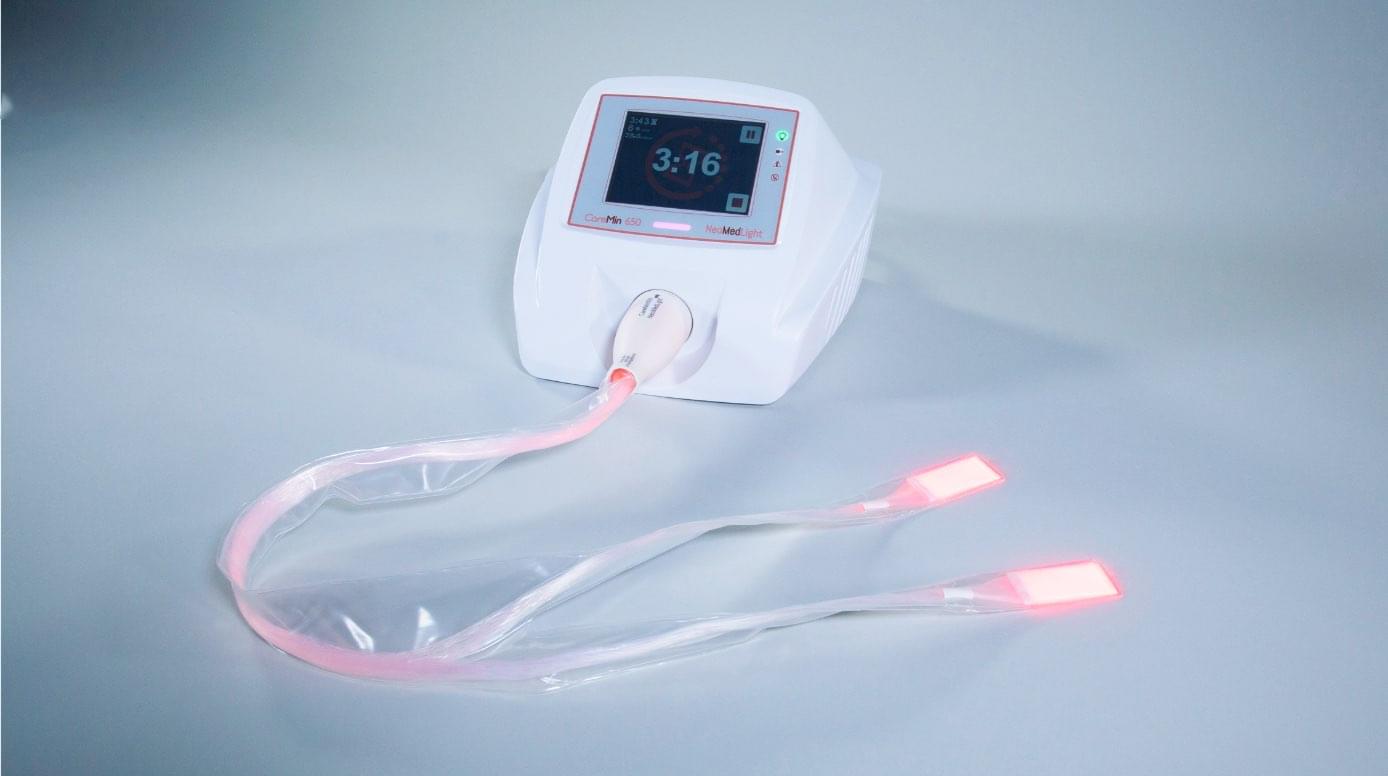
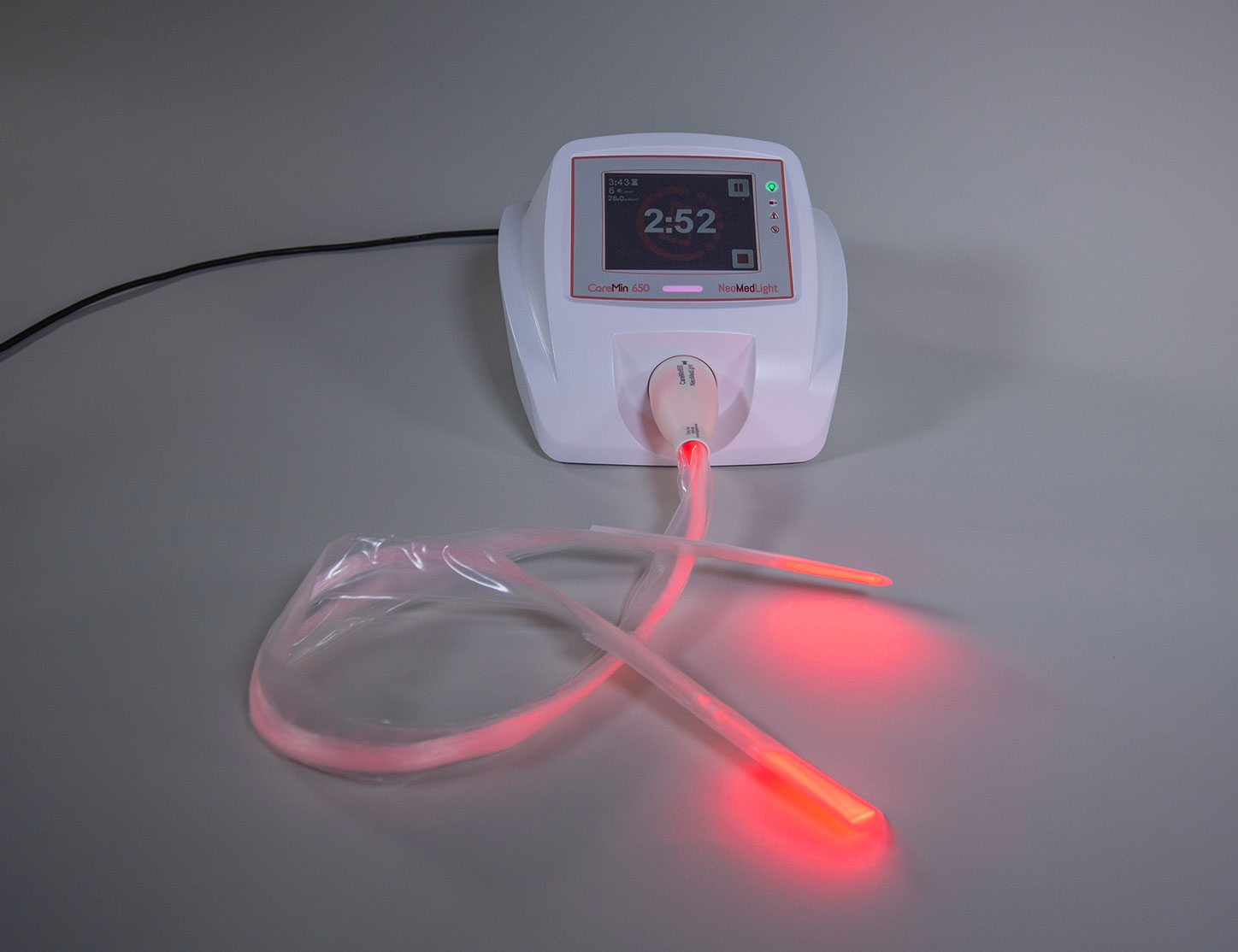
CareMin650™, a new photobiomodulation device for the prevention and treatment of radiation-induced mucositis and dermatitis: results of the prospective Safe PBM study. …
Thanks to its front-line anatomic medical devices, NeoMedLight will:
NeoMedLight is certified ISO 13485.
NeoMedLight is engaged in treating and preventing mucositis and dermatitis, a huge unmet clinical need.
An unrivalled medical device for severe cancer treatment complications: exact & easy red-LED photobiomodulation for mucositis and dermatitis.
View our published materials regarding phototherapy, research and our medical devices. Besides these studies, you can also access different training videos.


CareMin650™, a new photobiomodulation device for the prevention and treatment of radiation-induced mucositis and dermatitis: results of the prospective Safe PBM study. …

The Institute of Physics and Engineering in Medicine (IPEM) is a professional organization. It is composed of physicists, engineers ans technologists. Members …

RoseUp is an association that supports, informs and defends the rights of women affected by cancer. This association was founded by patients …
NML aims to change the field of phototherapy with a novel way to deliver therapeutic light. Based on the company unique approach to emitting light, PhotoBioModulation, a novel way to heal using light, will develop into a front line therapeutic tool for specialty wound care.
After its first step in neonatal jaundice, NeoMedLight’s primary focus is the treatment trough photobiomodulation of mucositis and dermatitis induced by chemo/radiotherapy.
Once established in that field, NeoMedLight will expend the field of indication and the range of therapeutic protocols to reach into other specialty wound care such as pressure ulcers, or diabetic wounds.
NeoMedLight has conceived and developed fiber-optic phototherapy devices to treat neonatal jaundice; as well as mucositis, dermatitis. Future projects include other specialty wound care such as pressure ulcers.
NeoMedLight is proud to be the recipient from a EU grant through the program H2020.
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement N°858849.
NeoMedLight is also an ISO 13485 certified company.
